John L. Gohres, Andrew T. Marin, Jie Lu , Charles L. Liotta, Charles A. Eckert “A Spectroscopic Investigation of Alkylcarbonic Acid Formation and Dissociation in CO2-Expanded Alcohols,” I&EC Res, 48, 1302-1306, 2009.
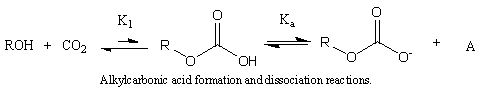
We show a technique to measure alkylcarbonic acid (ACA) formation and dissociation constants in CO2-expanded methanol, ethanol, and benzyl alcohol at 25 °C. In these systems, CO2 reacts with alcohols to form an in situ, self-neutralizing acid that exists under CO2 pressure. UV/vis spectroscopy is a noninvasive method to assay proton concentration, and the resulting values of this proton concentration yield effective equilibrium constants, the product of the formation, and dissociation constants. To separate the acid dissociation constant from the formation equilibrium constant, we employ a method using mass balances. From this, we find that methylcarbonic acid has a pKa of 5.7 and the relative ACA strengths are methyl > ethyl > benzyl. This work provides key parameters to design processes and expanded solvents that fully exploit the environmental and processing benefits of ACAs.
Megan E. Donaldson, Veronica Llopis Mestre, Daniele Vinci, Charles L. Liotta, and Charles A. Eckert, “Switchable Solvents for In-Situ Acid-Catalyzed Hydrolysis of β-Pinene,” I&EC Res, 48, 2542-2547, 2009..
Conventional acid-catalyzed reactions use homogeneous catalysts that generate large amounts of waste due to neutralization processes. We can eliminate such waste with the use of switchable solvents such as butadiene sulfone and piperylene sulfone, which can act as reaction media and generate reversible, in-situ acid catalysts. These solvents enable separation through use of a thermal switch, which shifts the equilibrium from the nonvolatile, polar aprotic solvent to highly volatile products. This equilibrium generates sulfurous acid through the addition of water, enabling acid-catalyzed reactions and easy separation due to the unstable nature of the acid at reversal temperatures. In this work, we use these switchable solvents to complete the hydrolysis of β-pinene to α-terpineol. We show a kinetic study of the hydrolysis reaction, illustrating high activity and selectivity in the novel reaction media. Further, we illustrate recycle of the solvent and catalyst five times without loss in activity or need for neutralization processes.
Megan E. Donaldson, Laura C. Draucker, Vittoria Blasucci, Charles L. Liotta, Charles A. Eckert, “Liquid- Liquid Equlibria of Polyethylene Glycol (PEG) 400 and CO2 with Common Organic Solvents,” Fluid Phase Equil., 277, 81-86, 2009.
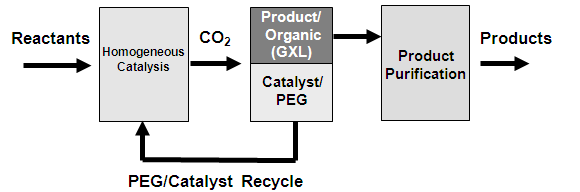
Liquid polyethylene glycol (PEG), in combination with carbon dioxide (CO2) and common organic solvents, enables the coupling of a homogeneous reaction with a heterogeneous separation. This is important for the application of homogenous catalysts, which offer superior reactivity but are difficult to separate and recycle. CO2 can act as a miscibility switch to shift the system from homogeneous at atmospheric conditions to heterogeneous under CO2 pressure. This allows for extraction of the products into the organic solvent phase and immobilization of the homogeneous catalyst in the PEG phase. This work examines the phase behavior of PEG and carbon dioxide with 1,4-dioxane and acetonitrile at 25 and 40 °C and pressures ranging from 5 to 8 MPa. The experimental data are compared to theoretical calculations using the Sanchez–Lacombe equation of state.
John L. Gohres, Alexander V. Popov, Rigoberto Hernandez, Charles L. Liotta, Charles A. Eckert, “Molecular Dynamics Simulations of Solvation and Solvent Reorganization Dynamics in CO2-Expanded Methanol and Acetone,” Journal of Chemical Theory Computation, 5, 267 – 275, 2009.
Composition-dependent solvation dynamics around the probe coumarin 153 (C153) have been explored in CO2-expanded methanol and acetone with molecular dynamics (MD) simulations. Solvent response functions are biexponential with two distinct decay time scales: a rapid initial decay (~0.1 ps) and a long relaxation process. Solvation times in both expanded solvent classes are nearly constant at partition compositions up to 80% CO2. The extent of solvation beyond this composition has the greatest tunability and sensitivity to bulk solvent composition. Solvent rotational correlation functions (RCFs) have also been used to explore rotational relaxation. Rotations have a larger range of time scales and are dependent on a number of factors including bulk composition, solvent-solvent interactions, particularly hydrogen bonding, and proximity to C153. The establishment of the solvation structure around a solute in a GXL is clearly a complex process. With respect to the local solvent domain around C153, it was seen to be primarily affected by a nonlinear combination of the rotational and diffusive transport dynamics.
Vittoria Blasucci, Cerag Dilek, Hillary Huttenhower, Ejae John, Veronica Llopis- Mestre, Pamela Pollet, Charles A. Eckert, and Charles L. Liotta, “One Component, Switchable, Neutral to Ionic Liquid Solvents Derived from Siloxylated Amines,” Chem Comm, 116-119, 2009.
A new class of one-component, thermally reversible, neutral to ionic liquid solvents derived from siloxylated amines is presented and characterized.

Pamela Pollet, Elizabeth D. Cope, Michelle K. Kassner, Reagan Charney, Stuart H. Terrett, Kent W. Richman, Bill Dubay, Joy Stringer, Charles A. Eckert, and Charles L. Liotta, “Production of ((1-Benzyl-3-diazo-2-oxo-propyl)-carbamic acid tert-butyl ester), a Diazoketone Pharmaceutical Intermediate, Employing a Small Scale Continuous Reactor,” I&EC Res, 48, 7032-7036, 2009.
N-Boc-(1S)-benzylhydroxy-3-chloropropylamine (6) is a precursor to pharmaceutically active compounds that act as human immunodeficiency virus (HIV) protease inhibitors. It is currently being produced via a batch process which includes a homologation step with diazomethane. This article considers the challenges faced when converting a traditional batch process to a continuous flow system for the production of the key intermediate (S)-1-benzyl-3-diazo-2-oxopropylcarbamic acid tert-butyl ester (4). A continuous flow reactor was designed, built, and used to carry out a two step reaction sequence: the formation of a temperature sensitive mixed anhydride intermediate (3) and the subsequent reaction of that intermediate (3) with trimethylsilyldiazomethane (8) to yield the diazoketone intermediate (4). By modifying the chemistry and maximizing the mixing and heat transfer, the batch process was successfully converted to a continuous flow process.
|
